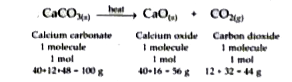Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL REACTIONS AND EQUATIONS
MTG IIT JEE FOUNDATION|Exercise SOLVED EXAMPLES|18 VideosCHEMICAL REACTIONS AND EQUATIONS
MTG IIT JEE FOUNDATION|Exercise NCERT SECTION|47 VideosCHEMICAL EQUILIBRIUM
MTG IIT JEE FOUNDATION|Exercise EXERCISE (INTEGER/NUMERICAL VALUE TYPE)|5 VideosFOOTSTEPS TOWARDS (NEET)
MTG IIT JEE FOUNDATION|Exercise MCQ|45 Videos
Similar Questions
Explore conceptually related problems
MTG IIT JEE FOUNDATION-CHEMICAL REACTIONS AND EQUATIONS-OLYMPIAD/HOTS CORNER
- What information is conveyed by the following equation ? CaCO(3) (s...
Text Solution
|
- When you mix solutions of lead (II) nitrate and potassium iodide. (i...
Text Solution
|
- Mg+CuOtoMgO+Cu Which of the following is wrong relating to the above...
Text Solution
|
- Which of the following are exothermic processes? 1. Reaction of wate...
Text Solution
|
- In the double displacement reaction between aqueous potassium iodide a...
Text Solution
|
- Four students A,B,C and D noted the initial colour of the solutions in...
Text Solution
|
- For the given redox reaction : Identify p, q, r and s.
Text Solution
|
- Identify the type of reaction for each of the following as Combinati...
Text Solution
|
- Identify the correct oxidant and reductant in the following reaction :...
Text Solution
|
- The given reaction occurs in a car battery when it is used to produce ...
Text Solution
|
- Rohit and Mayuri conducted two experiments to study the types of chemi...
Text Solution
|
- Which one of the following reactions is not possible?
Text Solution
|
- Addition of HCl to an aqueous solution of Pb(NO(3))(2) gives a
Text Solution
|
- The equation, Mg((s))+CuO((s))toMgO((s))+Cu((s)) represents (A) Deco...
Text Solution
|
- The given diagram shows the energy levels of the reactants and product...
Text Solution
|
- The chemical reaction between quicklime and water is characterised by ...
Text Solution
|
- The chemical reactions and their corresponding observable features are...
Text Solution
|
- A science teacher wrote the following statements about rancidity: (i...
Text Solution
|
- Which one is an example of a redox reaction?
Text Solution
|
- Classify each of the following reactions.
Text Solution
|
- P, Q and R are three metals that undergo chemical reactions as follows...
Text Solution
|