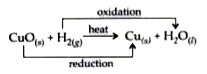
Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL REACTIONS AND EQUATIONS
MTG IIT JEE FOUNDATION|Exercise EXERCISE (MULTIPLE CHOICE QUESTIONS) (LEVEL-1)|30 VideosCHEMICAL REACTIONS AND EQUATIONS
MTG IIT JEE FOUNDATION|Exercise EXERCISE (MULTIPLE CHOICE QUESTIONS) (LEVEL-2)|20 VideosCHEMICAL REACTIONS AND EQUATIONS
MTG IIT JEE FOUNDATION|Exercise SOLVED EXAMPLES|18 VideosCHEMICAL EQUILIBRIUM
MTG IIT JEE FOUNDATION|Exercise EXERCISE (INTEGER/NUMERICAL VALUE TYPE)|5 VideosFOOTSTEPS TOWARDS (NEET)
MTG IIT JEE FOUNDATION|Exercise MCQ|45 Videos
Similar Questions
Explore conceptually related problems
MTG IIT JEE FOUNDATION-CHEMICAL REACTIONS AND EQUATIONS-NCERT SECTION
- Give an example of a double displacement reaction (only) reaction with...
Text Solution
|
- Identify the substances that are oxidized and the substances that are ...
Text Solution
|
- Identify the substances that are oxidised and the substances that are ...
Text Solution
|
- Which of the statements about the reaction below are incorrect? 2PbO...
Text Solution
|
- Fe(2)O(3) + 2Al rarr Al(2)O(3) + 2Fe The above reaction is an exampl...
Text Solution
|
- What happens when dilute hydrochloric acid is added to iron fillings? ...
Text Solution
|
- What is a balanced chemical equation? Why should chemical equations be...
Text Solution
|
- Translate the following statement into chemical equation and then bala...
Text Solution
|
- Translate the following statements into chemical equations and then ba...
Text Solution
|
- Translate the following statements into chemical equations and then ba...
Text Solution
|
- Translate the following statements into chemical equations and then ba...
Text Solution
|
- Balance the following chemical equations : HNO(3)+Ca(OH)(2)toCa(NO(3...
Text Solution
|
- Balance the following chemical equations : NaOH+H(2)SO(4)toNa(2)SO(4...
Text Solution
|
- Balance the following chemical equations : NaCl+AgNO(3)toAgCl+NaNO(3...
Text Solution
|
- Balance the following chemical equations : BaCl(2)+H(2)SO(4)toBaSO(4...
Text Solution
|
- Write the balanced chemical equations for the following reactions : ...
Text Solution
|
- Write the balanced chemical equation for the following reaction: Zin...
Text Solution
|
- Write the balanced chemical equations for the following reactions : ...
Text Solution
|
- Write the balanced chemical equations for the following reactions : ...
Text Solution
|
- Write the balanced chemical equations for the following and identify t...
Text Solution
|