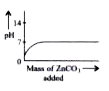
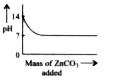
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MTG IIT JEE FOUNDATION-ACIDS , BASES AND SALTS -Olympiad /HOTS Corner
- What of the following options shows the correct arrangement of diffe...
Text Solution
|
- Four solutions labelled as P , Q , R and S have pH values 1 , 9,3 and...
Text Solution
|
- An element which reacts with water to form a solution which turns ph...
Text Solution
|
- Daivik , a class 10 student studied the reaction between a carbonat...
Text Solution
|
- The atmosphere of venus is made up of thick white and yellowish clo...
Text Solution
|
- 0.1 mol of a basic substance (X) requires 25cm^(3) of 8.0 mol//dm...
Text Solution
|
- Identify the wrong statement .
Text Solution
|
- The discomform caused by indigestion due to overeasting can be cured b...
Text Solution
|
- Which of the following graphs shows the change in pH when zinc carbo...
Text Solution
|
- Plaster of Paris is
Text Solution
|
- The shining finish to the walls is given by
Text Solution
|
- Which of the following compounds is alkaline in aqueous medium ?
Text Solution
|
- This does not possess water of crystallization
Text Solution
|
- A is an aqueous solution of Acid and B is an aqueous solution of base....
Text Solution
|
- Which of the following solutions has the lowest pH value ?
Text Solution
|
- In an experiment , 5 cm^(3) of 1.0 mol//dm^(3) NaOH solution is grad...
Text Solution
|
- pH of different solutions are given in the table Arrange these...
Text Solution
|
- If a few drops of a concentrated acid accidentally spill over the hand...
Text Solution
|
- On passing CO(2) gas in excess in aqueous solution of sodium carbona...
Text Solution
|
- Which of the following is acidic in nature ?
Text Solution
|