A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
MOLE CONCEPT, STOICHIOMETRY AND BEHAVIOUR OF GASES
MTG IIT JEE FOUNDATION|Exercise EXERCISE (MATCH THE FOLLOWING)|5 VideosMOLE CONCEPT, STOICHIOMETRY AND BEHAVIOUR OF GASES
MTG IIT JEE FOUNDATION|Exercise EXERCISE (ASSERTION & REASON TYPE) |10 VideosMOLE CONCEPT, STOICHIOMETRY AND BEHAVIOUR OF GASES
MTG IIT JEE FOUNDATION|Exercise SOLVED EXAMPLES |13 VideosMETALS AND NON METALS
MTG IIT JEE FOUNDATION|Exercise Olympiad/HOTS Corner|20 VideosPERIODIC CLASSIFICATION OF ELEMENTS
MTG IIT JEE FOUNDATION|Exercise Olympiad/HOTS Corner|15 Videos
Similar Questions
Explore conceptually related problems
MTG IIT JEE FOUNDATION-MOLE CONCEPT, STOICHIOMETRY AND BEHAVIOUR OF GASES -EXERCISE (MCQ)
- Which of the following is independent of temperature of a gas?
Text Solution
|
- The absolute temperature of a gas
Text Solution
|
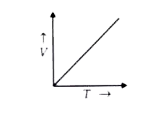
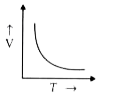
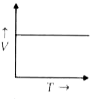
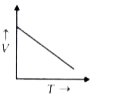
- Which of the following graphs relates V and T?
Text Solution
|
- The mass of carbon present in 0.5 mole of K(4)[Fe(CN)(6)] is -
Text Solution
|
- How is pressure related to volume at constant temperature?
Text Solution
|
- Equal volumes of different gases at any definite temperature and press...
Text Solution
|
- The value of gas contant per degree per mol is approximately :
Text Solution
|
- The total pressure of a mixture of two gases is
Text Solution
|
- A gas is found to have a formula [CO](x). If its vapour density is 70,...
Text Solution
|
- In the gas equation, PV = nRT
Text Solution
|
- Which one of the following plots will be a parabola at constant temper...
Text Solution
|
- The kinetic theory of gases predicts that total kinetic energy of a ga...
Text Solution
|
- Volume occupied by an ideal gas at one atmospheric pressure and 0^@C i...
Text Solution
|
- When gases are heated from 20^(@) to 40^(@) C at constant pressure the...
Text Solution
|
- In the equation of state of an ideal gas PV = nRT, the value of univer...
Text Solution
|
- If the absolute temperature of a gas is doubled and the pressure is re...
Text Solution
|
- The percentage of nitrogen in urea is about:
Text Solution
|
- To neutralise 100 mL of 0.1 N H(2)SO(4), amount of 2N NaOH required is
Text Solution
|
- A metal oxide contains 60% metal . The equivalent weight of metal is
Text Solution
|
- The simplest formula of a compound containing 50% of element X (atomic...
Text Solution
|