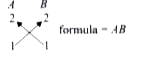A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING
MTG IIT JEE FOUNDATION|Exercise Exercise (Match the Following)|5 VideosCHEMICAL BONDING
MTG IIT JEE FOUNDATION|Exercise Exercise (Assertion & Reason Type)|10 VideosCHEMICAL BONDING
MTG IIT JEE FOUNDATION|Exercise Solved Examples |10 VideosCARBON AND ITS COMPOUNDS
MTG IIT JEE FOUNDATION|Exercise Olympaid/HOTS Corner|25 VideosCHEMICAL EQUILIBRIUM
MTG IIT JEE FOUNDATION|Exercise EXERCISE (INTEGER/NUMERICAL VALUE TYPE)|5 Videos
Similar Questions
Explore conceptually related problems
MTG IIT JEE FOUNDATION-CHEMICAL BONDING -Exercise (Multiple Choice Questions)
- Which of the following statements is not true about covalent compound...
Text Solution
|
- Element X is strongly electropositive and element Y is strongly electr...
Text Solution
|
- The number of lone pair of electrons on the central atom of XeF4 mol...
Text Solution
|
- Element A has 2 electrons in the outermost orbit and element B has 6 e...
Text Solution
|
- Which of the following has the highest dipole moment?
Text Solution
|
- In which solvent NaCl has maximum solubility ?
Text Solution
|
- Lattice energy of BeCO(3)(I), MgCO(3)(II) and CaCO(3)(III) is in order...
Text Solution
|
- Electronegativity values of elements help in predicting :
Text Solution
|
- The pair of elements that would likely to form an ionic bond is
Text Solution
|
- Which of the following compounds has a linear structure?
Text Solution
|
- The hybridisation of carbons involved in C-C single bond in CH-=C-CH =...
Text Solution
|
- The octer rule is not valid for the molecule .
Text Solution
|
- How is the bond in F, different from the bond in KCI?
Text Solution
|
- When a Cl atom gains an electron, it gets a charge of
Text Solution
|
- An ionic bond is formed between two atoms if the sum of their lattice ...
Text Solution
|
- When electrons are contributed by one atom but shared by both the ato...
Text Solution
|
- Identify the correct Lewis symbol from the following
Text Solution
|
- The total number of electrons that take part in forming bond in O2 is
Text Solution
|
- Carbon suboxide (C3O2) has recently been shown as a component of the ...
Text Solution
|
- Which of the following statements is incorrect?
Text Solution
|