A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MTG IIT JEE FOUNDATION-FOOTSTEPS TOWARDS (NEET) -MCQ
- A student takes about 5 mL distilled water in four test tubes marked P...
Text Solution
|
- In the reaction, Br(2) +2I^(-) to 2Br^(-) +I(2), the oxidising agent ...
Text Solution
|
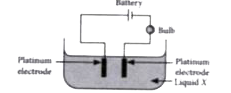
- An experiment was carried out using the apparatus shown below: ...
Text Solution
|
- Which of the following elements is not a metal?
Text Solution
|
- When 1.0 mol of compound Q is burnt in excess oxygen, 2.0 mol of carbo...
Text Solution
|
- Which of the following statements is correct about elements ""(9)^(19)...
Text Solution
|
- Observe the given figure and answer the question that follows: ...
Text Solution
|
- Acids like lactic acid, uric acid which are obtained usually from plan...
Text Solution
|
- Arrange the following atoms in the order of increasing atomic radius :...
Text Solution
|
- Electrolytic reduction method is used in the extraction of
Text Solution
|
- The given diagram represents a reaction.
Text Solution
|
- Change of Na(2)CO(3).10H(2)O to Na(2)CO(3).H(2)O on exposure to air is...
Text Solution
|
- The structural formulae of compounds Q and R are shown below: W...
Text Solution
|
- The liquid non-metal is
Text Solution
|
- Which of the following elements will form an acidic oxide?
Text Solution
|
- Consider the following reaction: (I) and (II) are respectively
Text Solution
|
- Moist sodium bicarbonate was placed on a strip of pH paper. The colour...
Text Solution
|
- Which of the following pairs of compounds have similar chemical proper...
Text Solution
|
- Which one of the following types of ores can be converted into oxide b...
Text Solution
|
- Which of the following processes does not involve either oxidation or ...
Text Solution
|