A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MTG IIT JEE FOUNDATION-FOOTSTEPS TOWARDS (JEE MAIN) -Integer/Numerical Value Type
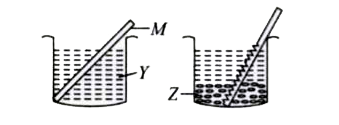
- A metal rod M was dipped in a coloured solution Y. After some time it ...
Text Solution
|
- From the given list, how many pairs of elements form ionic bonds when ...
Text Solution
|
- An element X belongs to group 14 and 2^(nd) period of the periodic tab...
Text Solution
|
- The pH of two solutions A and B are 2 and 4 respectively. What is the ...
Text Solution
|
- Copper(II) oxide reacts with ammonia to give copper, water and nitroge...
Text Solution
|
- CH(3)CH(2)OH overset("Ethanoic acid")toCompound, Z Number of hydrog...
Text Solution
|