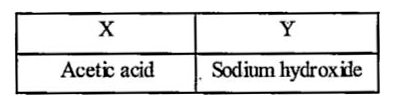
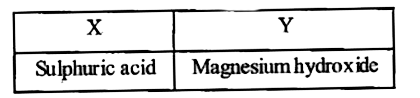A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- A substances X is sour to taste. Another substance Y was added to X. O...
Text Solution
|
- A mixture containing substance 'X' and 'Y' is mixed with a substance '...
Text Solution
|
- there are three substance X,Y and Z , the substance X, does not have a...
Text Solution
|
- When two substances X and Y were made to combine, another substance Z ...
Text Solution
|
- 1) Which of the above substance is a strong acid ? 2) Which of the a...
Text Solution
|
- n g of substance X reacts with m g of substance Y to from p g of subst...
Text Solution
|
- ................. अभिक्रिया में दो या दो से अधिक पदार्थ मिलकर नया पदार...
Text Solution
|
- Which is not formed by the physical mixture of two substances ?
Text Solution
|
- The new substances formed in the neutralization process are
Text Solution
|