Similar Questions
Explore conceptually related problems
Recommended Questions
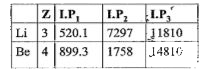
- I.P (in KJ/mole) of Li and Be are given below: I.P(2) of Li is mu...
Text Solution
|
- The tendency for catenation is much higher for C than for Si.
Text Solution
|
- The I.P(1) 's of N,P,O & S are in the order of
Text Solution
|
- NH(3) has a much higher bp than PH(3) because
Text Solution
|
- In........plants, the CO(2) compensation point is usually much higher ...
Text Solution
|
- ग्लिसरॉल की श्यानता एल्कोहॉल की तुलना में बहुत अधिक होती है क्यों ?
Text Solution
|
- Li की द्वितीय आयनन ऊर्जा He की अपेक्षाकृत अत्यधिक होती है ।
Text Solution
|
- कारण देते हुए समझाइये की कार्बनिक यौगिकों की संख्या अकार्बनिक यौगिकों ...
Text Solution
|
- The first ionization enthalpy of magnesium is higher than that of sodi...
Text Solution
|