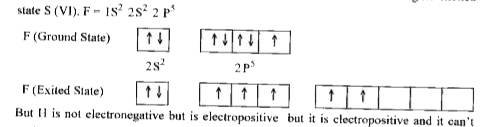
Text Solution
Verified by Experts
Topper's Solved these Questions
P-BLOCK ELEMENTS
ACCURATE PUBLICATION|Exercise P-BLOCK GROUP-17 (HALOGEN FAMILY )(2 OR 5 MARK QUESTIONS)|25 VideosP-BLOCK ELEMENTS
ACCURATE PUBLICATION|Exercise P-BLOCK ELEMENTS GROUP-18 (NOBLE GASES )(2 OR 5 MARK QUESTIONS)|17 VideosP-BLOCK ELEMENTS
ACCURATE PUBLICATION|Exercise P-BLOCK ELEMENTS GROUP-18 (NOBLE GASES )(1 MARK QUESTIONS)|9 VideosORGANIC COMPOUNDS CONTAINING NITROGEN COMPOUNDS
ACCURATE PUBLICATION|Exercise 2 OR 4 MARKS QUESTIONS|27 VideosSOLUTIONS
ACCURATE PUBLICATION|Exercise NUMERICAL QUESTIONS (3 MARKS)|31 Videos
Similar Questions
Explore conceptually related problems
ACCURATE PUBLICATION-P-BLOCK ELEMENTS-P-BLOCK (GROUP-16) (OXYGEN FAMILY )(2 OR 5 MARK QUESTIONS)
- Sulphur show +4 and +6 oxidation stae in their compounds but oxygen ca...
Text Solution
|
- H2S is a gas while H2O is liquid at room temperature·? Why ?
Text Solution
|
- Why SF6 is known but SH6 is not known ?
Text Solution
|
- Explain that SO2 can act as an oxidising agent as well as a reducing a...
Text Solution
|
- How would you account for the following : Sulphur has a great tendency...
Text Solution
|
- Oxygen gas is inert at room temperature why?
Text Solution
|
- Comment on nature of two S-O bond formed in SO2 molecule. Are the two ...
Text Solution
|
- Why SF6 is known but OF6 is not known
Text Solution
|
- Elements of Group 16 generally show lower value of first ionisation en...
Text Solution
|
- Oxygen gas is inert at room temperature why?
Text Solution
|
- Milk to cheese is an example of change.
Text Solution
|
- What is the use of ozone layer?
Text Solution
|
- What is tailing of mercury ?
Text Solution
|
- Which form of sulphur shows paramagnetic behaviour and why ?
Text Solution
|
- Why conc. sulphuric acid is always diluted by adding sulphuric acid to...
Text Solution
|
- Explain the reducing character of 16th group.
Text Solution
|
- What is the shape of ozone molecule ?
Text Solution
|
- Sulphur hexafluoride is used as a gaseous electrical insulator. Explai...
Text Solution
|
- Discuss the favourble conditions for the manufacture of sulphur trioxi...
Text Solution
|
- Oleum is: H2S2O7, H2S2O6, H4S2O7, H3S2O7.
Text Solution
|