Text Solution
Verified by Experts
Topper's Solved these Questions
P-BLOCK ELEMENTS
ACCURATE PUBLICATION|Exercise P-BLOCK ELEMENTS GROUP-18 (NOBLE GASES )(2 OR 5 MARK QUESTIONS)|17 VideosP-BLOCK ELEMENTS
ACCURATE PUBLICATION|Exercise P-BLOCK (GROUP-16) (OXYGEN FAMILY )(2 OR 5 MARK QUESTIONS)|29 VideosORGANIC COMPOUNDS CONTAINING NITROGEN COMPOUNDS
ACCURATE PUBLICATION|Exercise 2 OR 4 MARKS QUESTIONS|27 VideosSOLUTIONS
ACCURATE PUBLICATION|Exercise NUMERICAL QUESTIONS (3 MARKS)|31 Videos
Similar Questions
Explore conceptually related problems
ACCURATE PUBLICATION-P-BLOCK ELEMENTS-P-BLOCK GROUP-17 (HALOGEN FAMILY )(2 OR 5 MARK QUESTIONS)
- What are freons ?
Text Solution
|
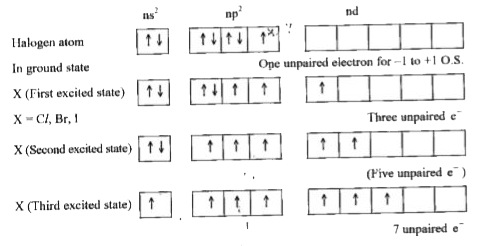
- Why fluorine shows-1 oxidation state only whereas other halogens show ...
Text Solution
|
- With what neutral molecule is CIO^- isoelectronic? Is that molecule a ...
Text Solution
|
- Account for the following: Among the halogens F2 is the strongest oxid...
Text Solution
|
- Account for the following: Why the acid strengths of halogen acids inc...
Text Solution
|
- Why does fluoriue show anomalous behaviour in its group ?
Text Solution
|
- SF6 is known but SCl6 is not known. Explain.
Text Solution
|
- Why are halogens coloured ?
Text Solution
|
- Why ClF3 exists, but FCl3 does not exist ?
Text Solution
|
- Why ICI is more reactive than I2 ?
Text Solution
|
- HF has higher boiling point than HCI.
Text Solution
|
- Draw the structure HCIO(4).
Text Solution
|
- Draw the structure of HCIO3?
Text Solution
|
- Draw the structure of HCIO3?
Text Solution
|
- Explain: Electron gain enthalpy of chlorine is more negative than fluo...
Text Solution
|
- Explain the following : Iodine is more soluble in KI solution than i...
Text Solution
|
- What are pseudohalogens ? Give example.
Text Solution
|
- Give three examples of pseudohalide ions.
Text Solution
|
- Halogens have maximum negative electron gain enthalpy in the respectiv...
Text Solution
|
- Write two uses of Chlorine.
Text Solution
|