Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ACCURATE PUBLICATION-D & F BLOCK ELEMENTS -2 OR 5 MARKS QUESTIONS
- A transition metal easily form alloys with other transition metals. Ex...
Text Solution
|
- Transition elements and their compounds are found to be good catalysts...
Text Solution
|
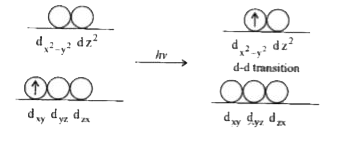
- Why transition element show coloured Ions ? Explain.
Text Solution
|
- Give the preparation of Potassium dichromate ,K2Cr2O7.
Text Solution
|
- Write the formula of ammonium hydroxide . Also name the elements pres...
Text Solution
|
- Write the formula of sodium sulphate . Also name the elements presents...
Text Solution
|
- Write the formula of carbon tetrachloride . Also name the elements pre...
Text Solution
|
- Define Lanthanoid Contraction and also explain cause of Lanthanoid con...
Text Solution
|
- Write the formula of potassium chloride . Also name the elements prese...
Text Solution
|
- Write the formula of calcium phosphate . Also name the elements presen...
Text Solution
|
- Write the formula of glucose. Also name the elements presents in them.
Text Solution
|
- Write the formula of chloroform. Also name the elements presents in t...
Text Solution
|
- Write the formula of bromoform. Also name the elements presents in the...
Text Solution
|
- Write the formula of hydrogen sulphide. Also name the elements present...
Text Solution
|
- Write the formula ammonium sulphate . Also name the elements presents ...
Text Solution
|
- Write the formula lime stone . Also name the elements presents in them...
Text Solution
|
- Write the formula of magnesium chloride . Also name the elements prese...
Text Solution
|
- Write the formula of lithium hydroxide . Also name the elements presen...
Text Solution
|
- The formula of water is H2O . Calculate its molecular mass.
Text Solution
|
- write formula of sodium oxide and also write elements present in them.
Text Solution
|