Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL REACTIONS AND EQUATIONS
SWAN PUBLICATION|Exercise ADDITIONAL IMPORTANT QUESTIONS(LONG ANSWER TYPE QUESTIONS)|10 VideosCHEMICAL REACTIONS AND EQUATIONS
SWAN PUBLICATION|Exercise ADDITIONAL IMPORTANT QUESTIONS(VERY SHORT ANSWER TYPE QUESTIONS)|8 VideosCARBON AND ITS COMPOUNDS
SWAN PUBLICATION|Exercise ADDITIONAL IMPORTANT QUESTIONS(LONG ANSWER TYPE QUESTIONS)|6 VideosMETALS AND NON METALS
SWAN PUBLICATION|Exercise ADDITIONAL IMPORTANT QUESTIONS ( Long Answer Type Questions )|9 Videos
Similar Questions
Explore conceptually related problems
SWAN PUBLICATION-CHEMICAL REACTIONS AND EQUATIONS-ADDITIONAL IMPORTANT QUESTIONS(SHORT ANSWER TYPE QUESTIONS)
- Write the chemical equation for the following reaction : Hydrogen su...
Text Solution
|
- Write a balanced equation for the following reaction : Methane burns i...
Text Solution
|
- White coloured silver chloride change to which colour in sunlight?
Text Solution
|
- Name the various chanbges taking place in nature.
Text Solution
|
- Why the surface of silver metal gets tarnished on exposure to air?
Text Solution
|
- Burning of paper is an example of change.
Text Solution
|
- Why is respiration considered as an exothermic reaction?explain
Text Solution
|
- A solution of potassium chloride when mixed with silver nitrate soluti...
Text Solution
|
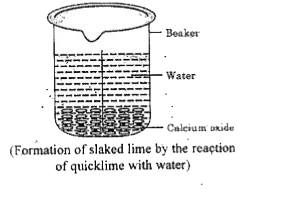
- Describe an activity to observe what happens when quick lime is added ...
Text Solution
|
- A magnesium ribbon burns with a dazzling flame in air (oxygen) and cha...
Text Solution
|
- What is oxidation reaction?
Text Solution
|