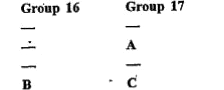Text Solution
Verified by Experts
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS
SWAN PUBLICATION|Exercise ADDITIONAL IMPORTANT QUESTIONS (Multiple Choice Questions)|7 VideosPERIODIC CLASSIFICATION OF ELEMENTS
SWAN PUBLICATION|Exercise ADDITIONAL IMPORTANT QUESTIONS (Very Short Answer Type Questions)|16 VideosPERIODIC CLASSIFICATION OF ELEMENTS
SWAN PUBLICATION|Exercise Textbook Questions |13 VideosMETALS AND NON METALS
SWAN PUBLICATION|Exercise ADDITIONAL IMPORTANT QUESTIONS ( Long Answer Type Questions )|9 Videos
Similar Questions
Explore conceptually related problems
SWAN PUBLICATION-PERIODIC CLASSIFICATION OF ELEMENTS -TEXTBOOK EXERCISES
- An element X has a valancy of 2. write the simplest formula for oxide ...
Text Solution
|
- Element 'X' forms a chloride with the formula XCl2 Which is a solid ...
Text Solution
|
- Calculate formula mass of following compound : K2O
Text Solution
|
- Which element has the electronic configuration 2, 8,4 ?
Text Solution
|
- Which element has the electronic configuration 2, 8,8, 1 ?
Text Solution
|
- Calculate the formula mass of KHCO3.
Text Solution
|
- Write the cation and anion present , if any , in the following : CH3CO...
Text Solution
|
- What property do all elements in the same column of the periodic table...
Text Solution
|
- Write the cation and anion present , if any , in the following : NaCl
Text Solution
|
- An atom has electronic configuration 2,8,7. What is the atomic number ...
Text Solution
|
- An atom has electronic configuration 2,8,7. To which of the following...
Text Solution
|
- The position of three elements A, B and C in the periodic table are as...
Text Solution
|
- The position of three elements A, B and C in the periodic table are a...
Text Solution
|
- Write the cation and anion present , if any , in the following : NH4NO...
Text Solution
|
- The position of three elements A, B and C in the Periodic Table are sh...
Text Solution
|
- Nitrogen(atomic number 7 ) and phosphorus (atomic number 15) belong to...
Text Solution
|
- How does the electronic configuration of an atom relate to its positio...
Text Solution
|
- In the modern periodic table, calcium (atomic number20) is surrounded ...
Text Solution
|
- Compare and contrast the arrangement of elements in mendeleev's period...
Text Solution
|