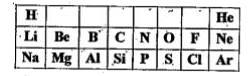Text Solution
Verified by Experts
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS
SWAN PUBLICATION|Exercise ADDITIONAL IMPORTANT QUESTIONS (Long Answer Type Questions)|16 VideosPERIODIC CLASSIFICATION OF ELEMENTS
SWAN PUBLICATION|Exercise ADDITIONAL IMPORTANT QUESTIONS (Very Short Answer Type Questions)|16 VideosMETALS AND NON METALS
SWAN PUBLICATION|Exercise ADDITIONAL IMPORTANT QUESTIONS ( Long Answer Type Questions )|9 Videos
Similar Questions
Explore conceptually related problems
SWAN PUBLICATION-PERIODIC CLASSIFICATION OF ELEMENTS -ADDITIONAL IMPORTANT QUESTIONS (Short Answer Type Questions)
- The element Be, Mg, Ca are placed in the second group of the periodic ...
Text Solution
|
- Table given below shows a part of the periodic table: Using this ...
Text Solution
|
- Table given below shows a part of the periodic table: Using this ...
Text Solution
|
- Table given below shows a part of the periodic table: Using this ...
Text Solution
|
- Explain the following term- Sericulture
Text Solution
|
- Two elements X and Y belong to group 1 and 2 - respectively in the sam...
Text Solution
|
- Two elements X and Y belong to group 1 and 2 - respectively in the sam...
Text Solution
|
- Two elements X and Y belong to group 1 and 2 - respectively in the sam...
Text Solution
|
- Two elements X and Y belong to group 1 and 2 - respectively in the sam...
Text Solution
|
- Two elements X and Y belong to group 1 and 2 - respectively in the sam...
Text Solution
|
- Two elements X and Y belong to group 1 and 2 - respectively in the sam...
Text Solution
|
- Lithium, sodium potassium are all metals that react with water to libe...
Text Solution
|
- Explain the following term- Rearing.
Text Solution
|
- An atom has electronic configuration 2,8,7. What is the atomic number ...
Text Solution
|
- The formula of magnesium oxide is Mgo. State the formula of barium nit...
Text Solution
|
- What is metalloid ?
Text Solution
|
- Fill in the blanks- Due to in clinical thermometer, mercury does not ...
Text Solution
|
- Fill in the blanks- is the solid wate collected from waste water duri...
Text Solution
|
- Write newland's law of octaves for classification of elements.
Text Solution
|
- Why Newland's law, is called "Law of Octaves."?
Text Solution
|