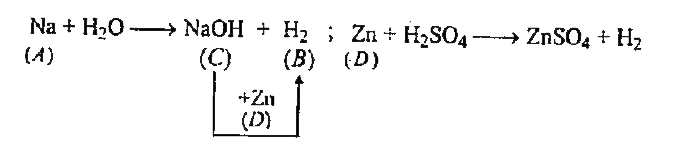A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
S-BLOCK ELEMENTS
BRILLIANT PUBLICATION|Exercise LEVEL-III (Multiple Choice Question)|10 VideosS-BLOCK ELEMENTS
BRILLIANT PUBLICATION|Exercise LEVEL-III (Numerical Type)|11 VideosS-BLOCK ELEMENTS
BRILLIANT PUBLICATION|Exercise LEVEL-II (ASSERTION-REASON TYPE)|20 VideosREDOX REACTIONS
BRILLIANT PUBLICATION|Exercise LEVEL -III|50 VideosSOME BASIC CONCAPTS OF CHEMISTRY
BRILLIANT PUBLICATION|Exercise LEVEL-III (Linked Comprehension Type)|6 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-S-BLOCK ELEMENTS-LEVEL-III (Single Correct Answer Type)
- Zinc on reaction with NaOH gives a salt (A) along with a gas (X) and (...
Text Solution
|
Text Solution
|
- When a substance A reacts with water it produces a combustible gas B a...
Text Solution
|
- The aqueous solution of an unknown sodium salt gives the following rea...
Text Solution
|
- Substances X,Y,Z and A are respectively
Text Solution
|
- An alkaline earth metal gives a salt with chlorine which is sparingly ...
Text Solution
|
- Find the final product of the reaction
Text Solution
|
- Which of the following statements is not correct? : Common salt absor...
Text Solution
|