A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PURIFICATION AND CHARACTERISATION OF ORGANIC COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL- III (Single Correct Answer Type)|7 VideosPURIFICATION AND CHARACTERISATION OF ORGANIC COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL- III (Multiple Correct Answer Type)|9 VideosPURIFICATION AND CHARACTERISATION OF ORGANIC COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL- III (Linked Comprehension Tyne (Paragraph I))|12 Videosp-BLOCK ELEMENTS
BRILLIANT PUBLICATION|Exercise LEVEL- III (Linked Comprehension Type) (Paragraph IV )|3 VideosREDOX REACTIONS
BRILLIANT PUBLICATION|Exercise LEVEL -III|50 Videos
BRILLIANT PUBLICATION-PURIFICATION AND CHARACTERISATION OF ORGANIC COMPOUNDS-LEVEL- II
- The ammonia evolved from the treatment of 0.30 g of an organic compoun...
Text Solution
|
- Analysis of organic compound (0.36 g) containing phosphorus gave 0.66 ...
Text Solution
|
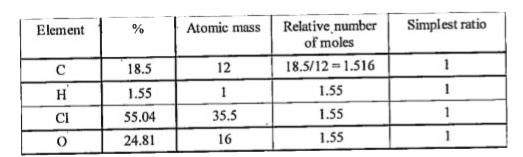
- If a compound on analysis was found to contain C = 18.5%, H = 1.55%, C...
Text Solution
|
- (5)/(19)g of an organic compound gave 22.4 cm^(3) of moist nitrogen me...
Text Solution
|
- An organic compound contains C, H and S. The minimum molecular weight ...
Text Solution
|
- Correct pair of compounds which gives blue colouration/precipitate and...
Text Solution
|
- In duma's method of estimation of nitrogen, 0.25 g of an organic compo...
Text Solution
|
- Which of the following will give blood red colour while doing Lassaign...
Text Solution
|
- A gaseous hydrocarbon gives upon combustion 0.72 g of water and 3.08 g...
Text Solution
|
- Ten millilitre of a gaseous hydrocarbon was burnt completely in 80 ml ...
Text Solution
|
- In the estimation of of nitrogen by Kjeldahl's method, 2.8 gm of an or...
Text Solution
|
- 0.3 gm of platinichloride of an organic diacidic base left 0.09 gm of ...
Text Solution
|
- A compound contains 38.8%C, 16%H, and 45.2%N. The formula of the compo...
Text Solution
|
- An organic compound on analysis gave C = 42.8%, H = 7.2%, and N = 50%....
Text Solution
|
- 0.14 gm of an acid required 12.5 ml of 0.1 N NaOH for complete neutral...
Text Solution
|
- 0.24 gm of a volatile liquid upon vaporisation gives 45 ml of vapours ...
Text Solution
|
- Liquid benzene (C(6)H(6)) burns in oxygen according to 2C(6)H(6)(l)+15...
Text Solution
|
- Some organic compounds are purified by distillation at low pressure be...
Text Solution
|
- Which is useful for separating benzoic acid from a mixture of benzoic ...
Text Solution
|
- 500 mL of a hydrocarbon gas burtn in excess of oxygen yielded 2500 mL ...
Text Solution
|