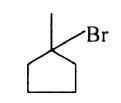
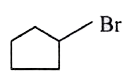
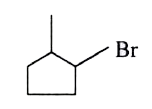
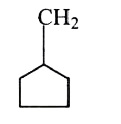
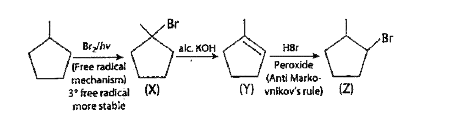A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS
BRILLIANT PUBLICATION|Exercise LEVEL-II (Assertion-Reason Type)|20 VideosHYDROCARBONS
BRILLIANT PUBLICATION|Exercise LEVEL-III (SINGLE CORRECT ANSWER TYPE)|20 VideosHYDROCARBONS
BRILLIANT PUBLICATION|Exercise LEVEL-III (LINKED COMPREHENSION TYPE)|9 VideosHYDROCARBON
BRILLIANT PUBLICATION|Exercise LEVEL II|48 VideosHYDROGEN
BRILLIANT PUBLICATION|Exercise LEVEL -III ( Linked Comprehension Type)|8 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-HYDROCARBONS-LEVEL-II
- During ozonolysis of CH2=CH2 if hydrolysis is made in absence of Zn du...
Text Solution
|
- In the following reaction, the major product is
Text Solution
|
- . The compound Z is
Text Solution
|
- The compound (i) decolourises KMnO4 (ii) forms ozonide with ozone and ...
Text Solution
|
- A hydrocarbon X adds on one mole of hydrogen to give hydrocarbon and d...
Text Solution
|
- Which of the following molecules/species are aromatic in character?
Text Solution
|
- In the reaction, C6H5CH3 overset"Oxidation"to A overset"NaOH"to B unde...
Text Solution
|
- Benzene contains double bonds but does not give addition reactions bec...
Text Solution
|
- The reaction of C6H5CH=CHCH3 with HBr produces: C6H5CH2underset(Br)un...
Text Solution
|
- Which product is formed when the following compound is treated with Br...
Text Solution
|
- The most stable conformation of the product of following reaction is:
Text Solution
|
- Provide the structure of the major product(s) from the following react...
Text Solution
|
- Which of the following reactions will not give propane? : , , ,
Text Solution
|
- What would be the product formed when 1-bromo-3-chlorocyclobutane reac...
Text Solution
|
- Out of the following compounds , I)Pent-1-ene , II)Pent-2-ene , III)2-...
Text Solution
|
- Which of the following will undergo faster dehydrobromination ?
Text Solution
|
- Match the following columns
Text Solution
|
- Give the decreasing order of reactivity of Diels-Alder reactions for t...
Text Solution
|
- 2-Phenyl propene on acidic hydration gives :
Text Solution
|
- The product (Y) is
Text Solution
|
 . The compound Z is
. The compound Z is