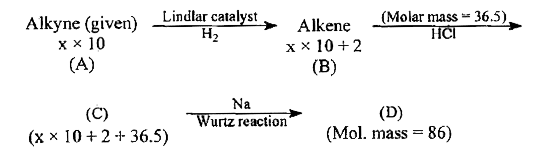Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS
BRILLIANT PUBLICATION|Exercise LEVEL-III (MATCHING COLUMN TYPE)|6 VideosHYDROCARBONS
BRILLIANT PUBLICATION|Exercise LEVEL-III (STATEMENT TYPE)|7 VideosHYDROCARBONS
BRILLIANT PUBLICATION|Exercise LEVEL-III (SINGLE CORRECT ANSWER TYPE)|20 VideosHYDROCARBON
BRILLIANT PUBLICATION|Exercise LEVEL II|48 VideosHYDROGEN
BRILLIANT PUBLICATION|Exercise LEVEL -III ( Linked Comprehension Type)|8 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-HYDROCARBONS-LEVEL-III (NUMERICAL TYPE)
- How many of the following on reductive ozonolysis will give only glyox...
Text Solution
|
- How many of the following species are aromatic in nature? cyclopenta...
Text Solution
|
- Which of the following molecules have zero dipole moment? cis-1, 2-d...
Text Solution
|
- how many alkenes are possible by the dehydrobromination of 3-bromo-3-c...
Text Solution
|
- An alkyne having molecular mass x xx 10 (A) is treated with Lindlar's ...
Text Solution
|
- How many eclipsed conformations are possible in butane?
Text Solution
|
- The number of pi-bonds in the product formed by passing acetylene thro...
Text Solution
|
- The number of meta directing groups among the following species are ...
Text Solution
|