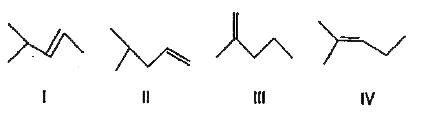
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-HYDROCARBONS-LEVEL-III (LINKED COMPREHENSION TYPE)
- 2-Phenyl propene on acidic hydration gives :
Text Solution
|
- Acid catalysed hydration of alkene gives alcohol. In this reaction add...
Text Solution
|
- Acid catalysed hydration of alkene gives alcohol. In this reaction add...
Text Solution
|
- Alkenes on catalytic hydrogenation give alkanes. The reactions are exo...
Text Solution
|
- Alkenes on catalytic hydrogenation give alkanes. The reactions are exo...
Text Solution
|
- Alkenes on catalytic hydrogenation give alkanes. The reactions are exo...
Text Solution
|
- The reaction given below is an example of Friedel-Craft alkylation rea...
Text Solution
|
- The reaction given below is an example of Friedel-Craft alkylation rea...
Text Solution
|
- The reaction given below is an example of Friedel-Craft alkylation rea...
Text Solution
|