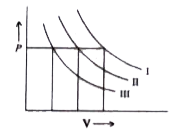
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER
BRILLIANT PUBLICATION|Exercise LEVEL II |55 VideosSTATES OF MATTER
BRILLIANT PUBLICATION|Exercise LEVEL III (Single Correct Answer Type)|10 VideosSTATES OF MATTER
BRILLIANT PUBLICATION|Exercise QUESTIONS |24 VideosSOME BASIC CONCEPTS OF CHEMISTRY
BRILLIANT PUBLICATION|Exercise LEVEL-I|55 VideosSTRUCTURE OF ATOMS
BRILLIANT PUBLICATION|Exercise LEVEL - III (Linked Comprehension Type)|8 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-STATES OF MATTER -LEVEL I
- At the top of the mountain, the thermometer reads 0^(@)C and the barom...
Text Solution
|
- Which of the following contains greatest number of N atoms?
Text Solution
|
- I, II and III are three isotherms, respectively, at T(1) ,T(2) , and T...
Text Solution
|
- At what temperature will hydrogen molecules have the same KE as Nitrog...
Text Solution
|
- The pressure exerted by 1 mol of CO(2) at 273 K is 34.98 atm. Assuming...
Text Solution
|
- A 3:2 molar mixture of N(2) and CO is present in a vessel at 500 bar p...
Text Solution
|
- What happened if a gas is expanded at constant temperature
Text Solution
|
- At 25^(@)C" and "730 mm pressure, 730 mL of dry oxygen was collected. ...
Text Solution
|
- At STP, the order of average velocity of molecules of H(2),N(2),O(2) a...
Text Solution
|
- An ideal gas obeying kinetic theory of gases can be liquified, if
Text Solution
|
- V vs T curves at different pressures P(1) and P(2) for an ideal gas ar...
Text Solution
|
- The vapour pressure of water at 80^(@)C" is "355 mm Hg. A one-litre ve...
Text Solution
|
- Boron forms a variety of unusual compounds with hydrogen. Achemist iso...
Text Solution
|
- A small quantity of gaseous NH(3)" and "HBr are introduced simultaneou...
Text Solution
|
- Which one of the following statement is not true about the effect of a...
Text Solution
|
- 2 moles of a gas confined in a 4 L flask exert a pressure of 9.0 atm o...
Text Solution
|
- 1 mol" of "N(2) gas at 0.8 atm takes 38s to diffuse through a pinhole,...
Text Solution
|
- What will be the molecular diameter of helium if van der Waals constan...
Text Solution
|
- Rate of diffusion of LPG (a mixture of n-butane and propane) is 1.25 t...
Text Solution
|
- Many laboratory gases are sold in steel cylinders with a volume of 43....
Text Solution
|