A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER
BRILLIANT PUBLICATION|Exercise LEVEL III (Single Correct Answer Type)|10 VideosSTATES OF MATTER
BRILLIANT PUBLICATION|Exercise LEVEL III (Multiple Correct Answer type )|14 VideosSTATES OF MATTER
BRILLIANT PUBLICATION|Exercise LEVEL I|50 VideosSOME BASIC CONCEPTS OF CHEMISTRY
BRILLIANT PUBLICATION|Exercise LEVEL-I|55 VideosSTRUCTURE OF ATOMS
BRILLIANT PUBLICATION|Exercise LEVEL - III (Linked Comprehension Type)|8 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-STATES OF MATTER -LEVEL II
- Dry ice (solid CO(2)) has occasionally been used as an explosive in m...
Text Solution
|
- Starting out on a trip into the mountains, you inflate the tires on yo...
Text Solution
|
- An open flask containing air is heated from 300 K to 500 K. What perce...
Text Solution
|
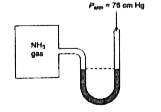
- A manometer attached to a flask contains ammonia gas have no differenc...
Text Solution
|
- A jar contains a gas and a few drops of water. The pressure in the jar...
Text Solution
|
- A gaseous mixture contains three gases A, B and C with a total number ...
Text Solution
|
- The density of a gas filled electric lamp is 0.75 kg"/"m^(3). After th...
Text Solution
|
- The average speed at temperature T^(@)C of CH(4)(g) is sqrt((28)/(88))...
Text Solution
|
- A gaseous mixture containing He, CH(4)" and "SO(2) was allowed to effu...
Text Solution
|
- The compressibility factor for nitrogen at 330 K and 800 atm is 1.90 a...
Text Solution
|
- 11 moles N(2) and 12 moles of H(2) mixture reacted in 20 litre vessel ...
Text Solution
|
- What is the density of wet air with 75% relative humidity at 1 atm and...
Text Solution
|
- A given volume of ozonized oxygen (containing 60% oxygen by volume) re...
Text Solution
|
- A mixture of nitrogen and water vapours is admitted to a flask at 760 ...
Text Solution
|
- If the slope of “Z' (compressibility factor) v/s “p'curve is constant ...
Text Solution
|
- For two samples A and B of ideal gas following curve is plotted betwee...
Text Solution
|
- Which one is not correct for gaseous state obeying van der Waals equat...
Text Solution
|
- Which is incorrect curve for Boyle's law?
Text Solution
|
- At 100^(@)C and 1 atm, if the density of liquid water is 1.0 g cm^(-3)...
Text Solution
|
- A mixture of NH(3) (g) and N(2) H(4) (g) is placed in a sealed contain...
Text Solution
|