A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL AND IONIC EQUILIBRIUM
BRILLIANT PUBLICATION|Exercise Level - III (Linked Comprehension Type Questions)|1 VideosCHEMICAL AND IONIC EQUILIBRIUM
BRILLIANT PUBLICATION|Exercise Level - III (Statement Type)|6 VideosCHEMICAL BONDING
BRILLIANT PUBLICATION|Exercise LEVEL-III ( Linked Comprehension Type )|12 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-CHEMICAL AND IONIC EQUILIBRIUM-Level - III (Linked Comprehension Type)
- Aqueous solution of phosphoric acid with a density of 1 g mL^(-1) cont...
Text Solution
|
- The dissociation of weak electrolyte (weak acid) is expressed in terms...
Text Solution
|
- Structure of phosphoric acid is : (pK(a1) = 2.12, pK(2) = 7.21 , pK...
Text Solution
|
- In Haber's process, ammonia is manufactured according to the following...
Text Solution
|
- In Haber's process, ammonia is manufactured according to the following...
Text Solution
|
- In Haber's process, ammonia is manufactured according to the following...
Text Solution
|
- 10 mole of NH(3) is heated at 15 atm from 27^(@C to 347^(@)C assuming ...
Text Solution
|
- 10 mole of NH(3) is heated at 15 atm from 27^(@C to 347^(@)C assuming ...
Text Solution
|
- 10 mole of NH(3) is heated at 15 atm from 27^(@C to 347^(@)C assuming ...
Text Solution
|
- The degree of dissociation of weak electrolyte is inversely proportion...
Text Solution
|
- The degre of dissociation of weak electrolyte is inversely proportiona...
Text Solution
|
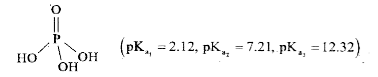 `(pK_(a1) = 2.12, pK_(2) = 7.21 , pK_(a3) = 12.32)`
`(pK_(a1) = 2.12, pK_(2) = 7.21 , pK_(a3) = 12.32)`