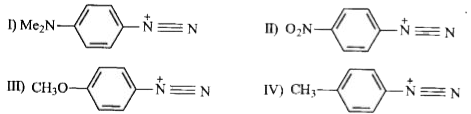A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-NITROGEN COMPOUNDS-LEVEL-III (Linked Comprehension Type)
- Mixture of two isomeric amines (C2 H7 N)A and B was treated with benze...
Text Solution
|
- Mixture of two isomeric amines (C2 H7 N)A and B was treated with benze...
Text Solution
|
- Mixture of two isomeric amines (C2 H7 N)A and B was treated with benze...
Text Solution
|
- Arene diazonium salts are more stable than alkanediazonium salts due t...
Text Solution
|
- Arene diazonium salts are more stable than alkanediazonium salts due t...
Text Solution
|
- Arene diazonium salts are more stable than alkanediazonium salts due t...
Text Solution
|
- All aliphatic amines are more basic than ammonia but due to delocaliza...
Text Solution
|
- All aliphatic amines are more basic than ammonia but due to delocaliza...
Text Solution
|
- All aliphatic amines are more basic than ammonia but due to delocaliza...
Text Solution
|