A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
POLYMERS, BIOMOLECULES AND CHEMISTRY IN EVERYDAY LIFE
BRILLIANT PUBLICATION|Exercise LEVEL -II|70 VideosPOLYMERS, BIOMOLECULES AND CHEMISTRY IN EVERYDAY LIFE
BRILLIANT PUBLICATION|Exercise LEVEL -III (Single Correct Answer Type)|9 VideosP-BLOCK ELEMENTS
BRILLIANT PUBLICATION|Exercise LEVEL-III|50 VideosREDOX REACTION & ELECTROCHEMISTRY
BRILLIANT PUBLICATION|Exercise QUESTION (ELECTROCHEMISTRY) (LEVEL -II) (ASSERTION-REASON)|3 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-POLYMERS, BIOMOLECULES AND CHEMISTRY IN EVERYDAY LIFE -LEVEL -III (Comprehension Type)
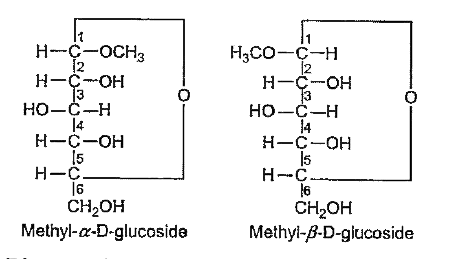
- Methyl- α-D-glucoside and methyl β-D-glucoside are : epimers, ano...
Text Solution
|
- Every amino acid has a carboxyl group and an amino group, and each gro...
Text Solution
|
- Every amino acid has a carboxyl group and an amino group, and each gro...
Text Solution
|
- . Form B is called
Text Solution
|
- Monosaccharides containing an aldehyde group are called aldoses while ...
Text Solution
|
- Monosaccharides containing an aldehyde group are called aldoses while ...
Text Solution
|
- Monosaccharides containing an aldehyde group are called aldoses while ...
Text Solution
|
- Vinyl chloride is the repeating unit in
Text Solution
|
- Which of the following are examples of thermoplastics?
Text Solution
|
- Which of the following are examples of homopolymers?
Text Solution
|
- Tranquillizers are drugs that reduce emotional instability, tension, f...
Text Solution
|
- Which of the following are non-narcotic analgesics?
Text Solution
|
- The bactericidal and bacteriostatic antibiotics, respectively, are
Text Solution
|