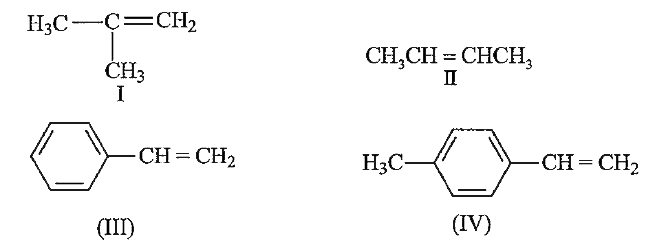
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
POLYMERS, BIOMOLECULES AND CHEMISTRY IN EVERYDAY LIFE
BRILLIANT PUBLICATION|Exercise LEVEL -III (Single Correct Answer Type)|9 VideosPOLYMERS, BIOMOLECULES AND CHEMISTRY IN EVERYDAY LIFE
BRILLIANT PUBLICATION|Exercise LEVEL -III (Multiple Correct Answer Type)|11 VideosPOLYMERS, BIOMOLECULES AND CHEMISTRY IN EVERYDAY LIFE
BRILLIANT PUBLICATION|Exercise LEVEL -III (Comprehension Type) |12 VideosP-BLOCK ELEMENTS
BRILLIANT PUBLICATION|Exercise LEVEL-III|50 VideosREDOX REACTION & ELECTROCHEMISTRY
BRILLIANT PUBLICATION|Exercise QUESTION (ELECTROCHEMISTRY) (LEVEL -II) (ASSERTION-REASON)|3 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-POLYMERS, BIOMOLECULES AND CHEMISTRY IN EVERYDAY LIFE -LEVEL -II
- Which cannot form addition polymers?
Text Solution
|
- Condensation polymers are formed from monomers
Text Solution
|
- Arrange the following in increasing order of reactivity towards cation...
Text Solution
|
- Natural rubber is not used in making footwear for polar regions becaus...
Text Solution
|
- Arrange the following polymers in the increasing order of their interm...
Text Solution
|
- The monomer of the polymer (-- CH2 - underset(CH3)underset(|) overset...
Text Solution
|
- Among cellulose, polyvinyl chloride, nylon and natural rubber, the pol...
Text Solution
|
- Which of the following statements about low density polythene is false...
Text Solution
|
- Acrilan is a hard, horny and a high melting material. Which of the fol...
Text Solution
|
- Which one of the following structures represents nylon 6,6 polymer?
Text Solution
|
- On complete hydrogenation, natural rubber produces
Text Solution
|
- Which among the following is monomer of neoprene?
Text Solution
|
- Which one of the following statements is not true?
Text Solution
|
- Buna-N synthetic rubber is a copolymer of
Text Solution
|
- Which one of the following sets forms the biodegradable polymer? : ...
Text Solution
|
- In which of the following polymers, ethylene glycol is one of the mono...
Text Solution
|
- The method preferred for determining the molecular weight of polymer i...
Text Solution
|
- SBR (GRS, Buna-S, Cold Rubber) is obtained by free radical copolymeris...
Text Solution
|
- Free radical polymerisation requires a free radical initiator. The mos...
Text Solution
|
- Which among the following statement is correct regarding polyurethanes...
Text Solution
|