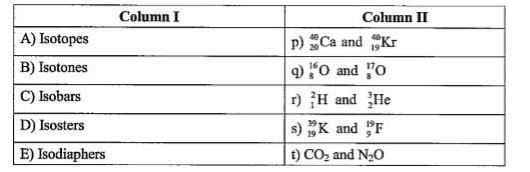
Text Solution
Verified by Experts
Topper's Solved these Questions
NUCLEAR CHEMISTRY
BRILLIANT PUBLICATION|Exercise LEVEL-III (Statement Type) |5 VideosNUCLEAR CHEMISTRY
BRILLIANT PUBLICATION|Exercise LEVEL-III (Linked Comprehension Type) |11 VideosNUCLEAR CHEMISTRY
BRILLIANT PUBLICATION|Exercise LEVEL-III (Numerical type) |6 VideosNITROGEN COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL-III (Linked Comprehension Type)|9 VideosP-BLOCK ELEMENTS
BRILLIANT PUBLICATION|Exercise LEVEL-III|50 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-NUCLEAR CHEMISTRY -LEVEL-III (Matching Column Type)
- Match the series (in Column I) with the end product (in Column II).
Text Solution
|
- Match the the Column I with the properties (one or more) in Column II...
Text Solution
|
- Match the Column I giving particles with the reaction involving these ...
Text Solution
|
- Match the nuclei/reaction with the process involved.
Text Solution
|
- Match the nuclei with their correct designation.
Text Solution
|