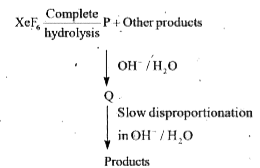
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P-BLOCK ELEMENTS
BRILLIANT PUBLICATION|Exercise LEVEL-II|50 VideosP-BLOCK ELEMENTS
BRILLIANT PUBLICATION|Exercise LEVEL-III|50 VideosP-BLOCK ELEMENTS
BRILLIANT PUBLICATION|Exercise LEVEL II (ASSERTION AND REASON)|20 VideosNUCLEAR CHEMISTRY
BRILLIANT PUBLICATION|Exercise LEVEL-III (Linked Comprehension Type) |11 VideosPOLYMERS, BIOMOLECULES AND CHEMISTRY IN EVERYDAY LIFE
BRILLIANT PUBLICATION|Exercise LEVEL -III (Comprehension Type) |12 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-P-BLOCK ELEMENTS-LEVEL-I
- What is X, in the reaction? KHSO4 + F2 to HF + X
Text Solution
|
- The correct order of increasing bond angles in the following species i...
Text Solution
|
- When Cl2 gas reacts with hot and concentrated sodium hydroxide solutio...
Text Solution
|
- Among the following which one is wrong-statement?
Text Solution
|
- In which of the following pairs , the two species are not isostructura...
Text Solution
|
- In which of the following pairs both the species are not isostructural...
Text Solution
|
- Which of the following species has equal number of sigma- and pi-bonds...
Text Solution
|
- Which of the following reactions of xenon compounds is not feasible?
Text Solution
|
- Under ambient conditions, the total number of gases released as produc...
Text Solution
|
- In which of the following arrangements, the order is not correct accor...
Text Solution
|
- Which one of the folowing does not have a pyramidal shape?
Text Solution
|
- The element that has the least tendency to show the inert-pair effect ...
Text Solution
|
- (NH4)2Cr2O7 on heating liberates a gas. The same gas will be obtained ...
Text Solution
|
- N2 forms NCI3 whereas P can form both PCl3 and PCl5. Why?
Text Solution
|
- Which of the following statements regarding sulphur is incorrect?
Text Solution
|
- The number of S-S bonds in sulphur trioxide trimer, (S3O9) is
Text Solution
|
- Hydrolysis of one mole of peroxydisulphuric acid produces
Text Solution
|
- Identify the incorrect statement among the following
Text Solution
|
- Which of the following xenon compounds may not be obtained by hydrolys...
Text Solution
|
- Which one of the following is the correct pair with respect to molecul...
Text Solution
|