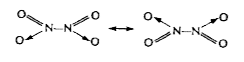
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P-BLOCK ELEMENTS
BRILLIANT PUBLICATION|Exercise LEVEL-III|50 VideosP-BLOCK ELEMENTS
BRILLIANT PUBLICATION|Exercise LEVEL-I|50 VideosNUCLEAR CHEMISTRY
BRILLIANT PUBLICATION|Exercise LEVEL-III (Linked Comprehension Type) |11 VideosPOLYMERS, BIOMOLECULES AND CHEMISTRY IN EVERYDAY LIFE
BRILLIANT PUBLICATION|Exercise LEVEL -III (Comprehension Type) |12 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-P-BLOCK ELEMENTS-LEVEL-II
- A substance which gives a yellow precipitate when boiled with an exces...
Text Solution
|
- Which one of the following statements regarding helium is incorrect?
Text Solution
|
- Which of the following statement is wrong?
Text Solution
|
- Conc. HNO3 stains skin and wool yellow because
Text Solution
|
- Nitrogen reacts with calcium and carbon or when N2 gas is passed over ...
Text Solution
|
- NH3 has pyramidal structure with HNH bond angle of 107^@. It forms com...
Text Solution
|
- Ordinary strong solution of HCl, HNO3 and H2SO4 contains roughly
Text Solution
|
- Dilute HNO3 cannot be concentrated beyond 68% by boiling because
Text Solution
|
- Paris green was used as a pigment due to unique light green colour but...
Text Solution
|
- Which one of the following halide does not hydrolyse
Text Solution
|
- Following tests are shown by (i) Decolourisation of acidified soln....
Text Solution
|
- PCl5 and PH3 exist but PH5 does not because
Text Solution
|
- Holme's signals produce burning gases which serve as a signal to the a...
Text Solution
|
- If phosphorous acid is allowed to react with excess of KOH, the produc...
Text Solution
|
- When NH4 OH is added to copper sulphate solution, blue colour is obtai...
Text Solution
|
- The number of P-O-P and P-OH bonds present respectively in pyrophospho...
Text Solution
|
- When ammonia is heated with CO2 under pressure, the product is
Text Solution
|
- White phosphorus reacts with caustic soda. The products are PH3 and Na...
Text Solution
|
- Nitrogen molecule is chemically less active because of its
Text Solution
|
- PCl5 is kept in well stoppered bottles because
Text Solution
|