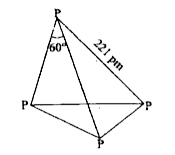
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P-BLOCK ELEMENTS
BRILLIANT PUBLICATION|Exercise LEVEL-III|50 VideosP-BLOCK ELEMENTS
BRILLIANT PUBLICATION|Exercise LEVEL-I|50 VideosNUCLEAR CHEMISTRY
BRILLIANT PUBLICATION|Exercise LEVEL-III (Linked Comprehension Type) |11 VideosPOLYMERS, BIOMOLECULES AND CHEMISTRY IN EVERYDAY LIFE
BRILLIANT PUBLICATION|Exercise LEVEL -III (Comprehension Type) |12 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-P-BLOCK ELEMENTS-LEVEL-II
- The BCl3 is a planar molecule whereas NCl3 is pyramidal because
Text Solution
|
- Hydrolysis of NCl3 gives NH3 and X. Which of the following is X?
Text Solution
|
- Atoms in P4 molcule of white phosphorus are arranged regularly in the ...
Text Solution
|
- One mole of H3PO3 on reaction with excess of NaOH gives:
Text Solution
|
- If O2 is removed from the formula of anhydride of HNO2, their the form...
Text Solution
|
- Which of the following is correct?
Text Solution
|
- The oxyacid of phosphorus in which phosphorus has the lowest oxidation...
Text Solution
|
- How many peroxy linkages are present in pyrophosphric acid?
Text Solution
|
- Which of the following options are true (T) and which are false (F) ? ...
Text Solution
|
- Which of the following series of fluorides is known?
Text Solution
|
- The compounds of S obtained by reaction of S and conc. hot KOH when re...
Text Solution
|
- Which of the following pseudohalides does not form dimer like halogen ...
Text Solution
|
- Which blue-liquid is obtained on reacting equimolar amounts of two gas...
Text Solution
|
- Select correct statement. (i) Mixture of NH4Cl and NaNO2 on heating...
Text Solution
|
- The true statement for the acids of phosphorus, 'H3PO2, H3PO3 and H3...
Text Solution
|
- NH4Cl(s) is heated in a test tụbe. Vapours are brought in contact with...
Text Solution
|
- The thermal stability of hydrides of oxygen family is in order :
Text Solution
|
- Which of the following can convert acidified Cr2O7^(2-) to green? SO2/...
Text Solution
|
- Bleaching of a fabric cloth is done using Aand excess of chlorine is r...
Text Solution
|
- Select incorrect statement
Text Solution
|