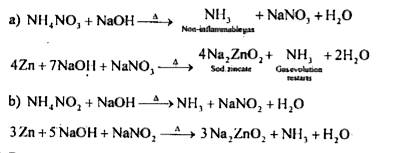
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-P-BLOCK ELEMENTS-LEVEL-III
- Which statements are correct?
Text Solution
|
- The nitrogen containing compound produced in the reaction of HNO3 with...
Text Solution
|
- A solution of colourless salt Honboiling with excess NaOH produces a n...
Text Solution
|
- The correct statement(s) about O3 is (are)
Text Solution
|
- Which of the following hydrogen halides react(s) with AgNO3 (aq) to gi...
Text Solution
|
- The correct statement(s) regarding (i) HClO, (ii) HClO2, (iii) HClO3 a...
Text Solution
|
- The nitrogen oxide(s) that contains(s) N-N bond(s) is (are)
Text Solution
|
- Which of the following statements are incorrect?
Text Solution
|
- Which of the following reactions are feasible?
Text Solution
|
- The noble gases which are lighter than air are
Text Solution
|
- Among the following, the number of compounds that can react with PCl5 ...
Text Solution
|
- Ozone reacts with dry iodine to form an oxide having ........... Oxyge...
Text Solution
|
- In the interhalogen compound AB(n) what is the maximum value of n?
Text Solution
|
- Chlorine water on cooling deposits greenish yellow crystals of formula...
Text Solution
|
- How many dpi-ppi bonds are there in XeO4?
Text Solution
|
- This section contains questions each with two columns-I and II. Match ...
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|