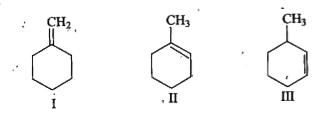
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
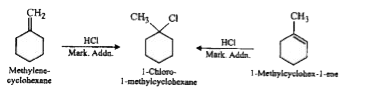
- In the reaction with HCl, an alkene reacts in accordance with the Mark...
Text Solution
|
- In the reaction with HCl, an alkene reacts in accordance with the Mark...
Text Solution
|
- In the reaction with HCl, an alkene reacts in accordance with the mark...
Text Solution
|
- In the reaction with HCl, an alkene reacts in accordance with Markowni...
Text Solution
|
- In the reaction with HCI, an alkene reacts in accordance with the Mark...
Text Solution
|
- In the reaction with HCl , an alkene reacts in accordance with the Mar...
Text Solution
|
- In the reaction with HCl, an alkene reacts in accordance with Markowni...
Text Solution
|
- In the reaction with HCl, an alkene reacts in accordance with the Mark...
Text Solution
|
- In the reaction with HCl , an alkane reacts in accordance with the Mar...
Text Solution
|