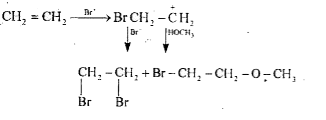A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In methyl alcohol solution, bromine reacts with ethylene to yield BrCH...
Text Solution
|
- Ethylene reacts with Br(2) to give 1,2-dibromoethane. The reaction pro...
Text Solution
|
- The IUPAC name of CH(3)OCH(2)CH(2)OCH(2)CH(2)OCH(2)CH(3) is
Text Solution
|
- In methyl alcohol solution, bromine reacts with ethyle (ethene) to yie...
Text Solution
|
- Prepare ethylene from : 1,2-dibromoethane
Text Solution
|
- The reaction conditions used for converting 1,2- dibromoethane to ethy...
Text Solution
|
- The reaction conditions used for converting 1,2- dibromoethane to ethy...
Text Solution
|
- In methyl alcohol solution, bromine reacts with ethyle (ethene) to yie...
Text Solution
|
- The reaction conditions used for converting 1,2-dibromoethane to ethyl...
Text Solution
|