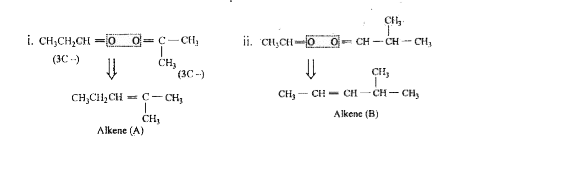
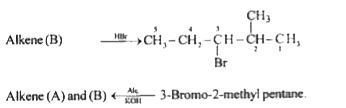A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A white precipitate was formed slowly when Ag NO3 was added-to a comp...
Text Solution
|
- A white precipitate was formed slowly when silver nitrate was added to...
Text Solution
|
- An aromatic compound 'A' on treatment with aqueous ammonia and heating...
Text Solution
|
- An aromatic compound 'A' on treatment with aqueous ammonia and heating...
Text Solution
|
- An aromatic compound 'A' on treatment with aqueous ammonia and heating...
Text Solution
|
- An orgnic compound (A) on reduction gave a compound (B). Upon treatmen...
Text Solution
|
- A white precipitate was formed slowly when AgNO(3) was added to a comp...
Text Solution
|
- An organic compound A with molecular formula C4H9 Br on treatment with...
Text Solution
|
- जब C6H13Cl अणुसूत्र वाले एक योगिक A को सिल्वर नाइट्रेट के साथ मिलाया ज...
Text Solution
|