A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Due to sp^(2)-hybridisation of C-atom holding the halogen atom, resona...
Text Solution
|
- C-Cl bond of chlorobenzene in comparison to C-Cl bond of methyl chlori...
Text Solution
|
- Assertion: It is difficult to replace chlorine by -OH in chlorobenzene...
Text Solution
|
- Aryl halides do not undergo nucleophilic substitution reactions under ...
Text Solution
|
- क्लोरो बेन्जीन में C-Cl आबंध मेथिल क्लोराइड में C-Cl आबंध की तुलना म...
Text Solution
|
- Pick out the correct statement 1. The C-Cl bond in Chlorobenzene is sh...
Text Solution
|
- The C-Cl bond in chlorobenzene as compared with C-Cl bond in methyl ch...
Text Solution
|
- Assertion: It is difficult to replace chlorine by -OH in chlorobenzene...
Text Solution
|
- Assertion (A) : It is difficult to replace chlorine by -OH in chlorobe...
Text Solution
|
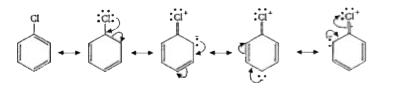
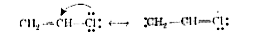 .
.