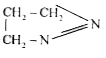
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Paragraph IV There are several factors which decide the fate of subs...
Text Solution
|
- Statement-1: In protic solvents phenoxide ion is alkylated primarily a...
Text Solution
|
- ऐल्किल हैलाइड में नाभिकस्नेही प्रतिस्थापन अभिक्रिया कैसे होती है ? इसक...
Text Solution
|
- तृतीयक ऐल्किल हैलाइड SN2 क्रियाविधि के प्रतिस्थापन के लिए व्यावहारिक र...
Text Solution
|
- Tertiary alkyl halide is practically inert to substitution by S(N) 2 m...
Text Solution
|
- The order of alkyl halides in nucleophilic substitution is
Text Solution
|
- In SN1 reactions, rate of reaction depends on a) Concentration of alky...
Text Solution
|
- Teritiary alkyl halides are practically inert to substitution by SN2 m...
Text Solution
|
- A dextrorotatory alkyl halide is allowed to react with nucleophile, le...
Text Solution
|