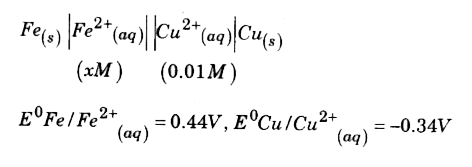A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- E("cell") = 0.78 volt for the following cell.
Text Solution
|
- For the redox change , Zn(s) + underset (0.1M)FuCu^2+ rarr underset...
Text Solution
|
- E^(@) (SRP) of different half cell given {:(E(Cu^(2+)//Cu)^(@) =0.34...
Text Solution
|
- Consider the following reaction, Zn(s)+Cu^(2+) (0.1 M) rarr Zn^(2+) ...
Text Solution
|
- In which of the following cell(s): E("cell")=E("cell")^(@) ?
Text Solution
|
- In which of the following (E("cell")-E("cell")^(@))=0
Text Solution
|
- In which case (E("cell")-E("cell")^(@)) is zero
Text Solution
|
- Consider the following reaction, Zn(s)+Cu^(2+) (0.1 M) rarr Zn^(2+) ...
Text Solution
|
- E(cell)=0.78 volt for the following cell. underset(Fe((s))|Fe((aq))^(2...
Text Solution
|