Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
OMEGA PUBLICATION-CHEMICAL KINETICS -MULTIPLE CHOICE QUESTIONS (MCQ s)
- The following results have been obtained during the kinetic studies of...
Text Solution
|
- Consider the chemical reaction, N (2) (g) + 3 H (2)(g) to 2 NH (3) (...
Text Solution
|
- For a reaction involving solid, decreasing which given below will incr...
Text Solution
|
- Rate of first order reaction depends upon
Text Solution
|
- Under a given set of experimental conditions, with increase of concent...
Text Solution
|
- Rate at which a substance reacts depends upon its
Text Solution
|
- RCOO R ' + Na OH to RCOO Na + R ' OH. What type of reaction is this ?
Text Solution
|
- Units of rate constant for the first and zero order reactions in terms...
Text Solution
|
- Units of specific reaction rate for 2nd order reaction is
Text Solution
|
- The units of rate constant for first order equation.
Text Solution
|
- The hydrolysis of ester in alkaline medium is a
Text Solution
|
- Given the unit of second order rate constant
Text Solution
|
- The half life period of a zero order reaction is independent of initia...
Text Solution
|
- What is the effect of temperature on rate of a reaction.
Text Solution
|
- The activation energy of reaction is equal to
Text Solution
|
- The activation energy of a reaction can be determined by
Text Solution
|
- The wavelength of first spectral line in the balmer series is 6561 ang...
Text Solution
|
- The substance that increases the speed of a chemical reactions is call...
Text Solution
|
- The reaction rate is found to depend upon two concentration terms. The...
Text Solution
|
- For which of the following , the units of r constant and rate of the r...
Text Solution
|
- A zero order reaction is one whose rate independent of
Text Solution
|
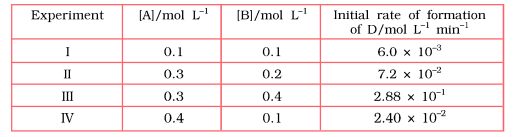 Determine the rate law and the rate constant for the reaction.
Determine the rate law and the rate constant for the reaction.