Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
OMEGA PUBLICATION-THE P-BLOCK ELEMENTS-MULTIPLE CHOICE QUESTIONS
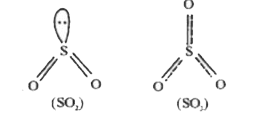
- Explain that SO2 can act as an oxidising agent as well as a reducing a...
Text Solution
|
- Bauxite containing chief impurities of oxides of silicon is called
Text Solution
|
- Thermite is a mixture of
Text Solution
|
- The first ionisation energy of silicon is lower than that of
Text Solution
|
- Account for the following: The +2 oxidation state of lead is more stab...
Text Solution
|
- Define catenation?
Text Solution
|
- Silicon hydrides are called
Text Solution
|
- C Cl4 is not hydrolysed but SiCl4 can be hydrolysed with water. Why ?
Text Solution
|
- The formula of dry ice is ………………….. .
Text Solution
|
- Which of the following is an ore of boron?
Text Solution
|
- Compounds of Boron with hydrogen are known as
Text Solution
|
- Diborane has
Text Solution
|
- In B(2)H(6) , B-atom is
Text Solution
|
- Orthoboric acid is
Text Solution
|
- Boron halides are
Text Solution
|
- Ammonia is, in general,
Text Solution
|
- NH(3) can be prepared by
Text Solution
|
- Boron trioxide can be reduced to boron with
Text Solution
|
- In reaction BF(3) + 3LiBH(4) rarr 3LiF+ X, X is
Text Solution
|
- Graphite is a good conductor of electricity because
Text Solution
|
- The laughing gas is
Text Solution
|