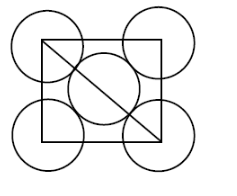
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-SOLID STATE-EXERCISE
- In a solid 'AB' having NaCl structure,'A' atoms occupy the corners of ...
Text Solution
|
- In which of the following crystals alternate tetrahedral voids are occ...
Text Solution
|
- The packing efficiency of the two dimensional square unit cell shown i...
Text Solution
|
- A compound Mp Xq has cubic close packing arrangement of X. its unit ce...
Text Solution
|
- If the unit cell of a mineral has cubic close packed array of oxygen a...
Text Solution
|
- Which of the following statement is not true about the hexagonl close ...
Text Solution
|
- Which of the following statements are correct?
Text Solution
|
- In which of the following structures, the coordination number of both ...
Text Solution
|
- Which of the following is not correct for Frenkel defect in crystals?
Text Solution
|
- The coordination number of eight for cation is found in
Text Solution
|
- Which of the following system do not give correct descrptin of axial l...
Text Solution
|
- The correct statement regarding defects in solids is
Text Solution
|
- With respect to graphite and diamond, which of the statements given is...
Text Solution
|
- Which type of defects are present in AgBr and ZnS crystal systems?
Text Solution
|
- The correct statement cubic close packed in three dimensional structur...
Text Solution
|
- Define the following term- Crop
Text Solution
|
- In the crystalline solids the smallest repeating part in the lattice i...
Text Solution
|
- In a cubic lattice of XYZ, X atoms are present at all corners except o...
Text Solution
|
- Can cubc lattice have end centred unit cell?
Text Solution
|
- The number of atoms per unit cell in BCC is
Text Solution
|