A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-SOLUTIONS -EXERCISE
- We have three aqueous solutions of NaCl labelled as 'A', 'B' and 'C' w...
Text Solution
|
- What type of interactions hold the molecules together in a polar molec...
Text Solution
|
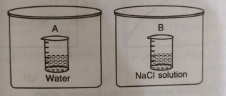
- Two beakers of capcatity 500 mL were taken one of these beakers labell...
Text Solution
|
- Components of a binary mixture of two liquids A and B were being separ...
Text Solution
|
- 4L of 0.02 M aqueous solution of NaCI was diluted by adding one litre ...
Text Solution
|
- (Tick the correct option) Rest mass of a photon
Text Solution
|
- KH Value for Ar(g),CO2(g), HCHO(g) and CH(4) (g) are 40.39, 1.67, 1.8...
Text Solution
|
- According to Henry' s law, the solubility of gas in a given volume of ...
Text Solution
|
- Which of the following statements are not true?
Text Solution
|
- Show that relative lowering in vapour pressure is a colligative pr...
Text Solution
|
- What is Van't Hoff factor ?
Text Solution
|
- Isotonic solutions must have the same
Text Solution
|
- Which of the following binary operation is commutative ?
Text Solution
|
- In isotonic solutions………
Text Solution
|
- Fill in the blanks- malaria is caused by .
Text Solution
|
- Colligative properties are observed when
Text Solution
|
- Match the following:
Text Solution
|
- Match the items given in column I with type of solutoins given in colu...
Text Solution
|
- Match the laws given in Column I with expression given in Column II.
Text Solution
|
- Match the terms given in Column I with expression given in Column II.
Text Solution
|