Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-SOLUTIONS -EXERCISE
- Match the items given in column I with type of solutoins given in colu...
Text Solution
|
- Match the laws given in Column I with expression given in Column II.
Text Solution
|
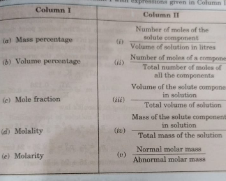
- Match the terms given in Column I with expression given in Column II.
Text Solution
|
- In the following questions a statement of assertion followed by a stat...
Text Solution
|
- In the following questions a statement of assertion followed by a stat...
Text Solution
|
- In the following questions a statement of assertion followed by a stat...
Text Solution
|
- State the conditions resulting in reverse osmosis?
Text Solution
|
- If 30 g a solute of molecular mass 154 is dissolved in 250 g of benzen...
Text Solution
|
- How will you calculate the molecular mass of a solute with the help of...
Text Solution
|
- What is the significance of van't Hoff factor?
Text Solution
|
- How is the molality of a solution different from its molarity?
Text Solution
|
- True or false Solution of ethanol and cyclohexane shows positive deriv...
Text Solution
|
- Why do gases always tend to be less soluble in liquids as the temperat...
Text Solution
|
- How many grams of ethylene glycol (molar mass = 62) should be added to...
Text Solution
|
- Sodium chloride solution freezes at lower temparature than water but b...
Text Solution
|
- True or false Elevation in boiling point of 0.1 m NaCl solution will b...
Text Solution
|
- A solution contains 0.8960 g of K2SO4 in 500 mL solution. Its osmotic ...
Text Solution
|
- Write differences between ideal and non-ideal solutions.
Text Solution
|
- Why do you get sometimes abnormal molecular mass of substances by usin...
Text Solution
|
- The aqueous solutions containing respectively 7.5 g of urea (molar mas...
Text Solution
|