Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-ELECTROCHEMISTRY-EXERCISE
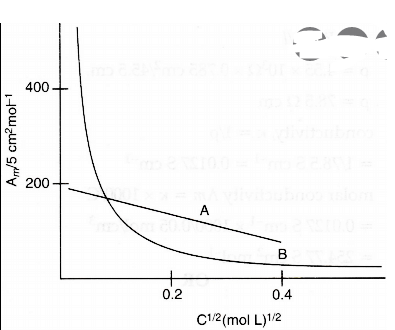
- The following curve is obtained when molar conductivty, Lambdam is plo...
Text Solution
|
- The resistance of a 0.25 mol or solution of an molar solution of an el...
Text Solution
|
- What is molar conductivity of an electrolyte? Solution containing 0.1 ...
Text Solution
|
- Explain the variation in molar conductivity of weak electrolyte with c...
Text Solution
|
- Calculate the resistance of 0.01N solution of an electrolyte whose equ...
Text Solution
|
- The resistance of a 0.5 M solution of an electrolyte in a conductivity...
Text Solution
|
- A conductance cell was filled with a 0.02 M KCl solution which has a s...
Text Solution
|
- The conductivity of a solution containing 1.0 g of anhydrous BaCl2 in ...
Text Solution
|
- The resistance of a 0.5M solution of an electrolyte was found to be 30...
Text Solution
|
- A conductance cell was filled with a 0.02 M KCl solution which has a s...
Text Solution
|
- When a certain conductance cell was filled 0.1 mol L^-1 KCl, it has a ...
Text Solution
|
- The resistance of a conductivity cell with 0.1 M KCl solution is found...
Text Solution
|
- The resistance of a conductivity cell with 0.1 M KCl solution is found...
Text Solution
|
- The molar conductivity of 0.04 M solution of MgCl2 is 200 Scm^3mol^(-1...
Text Solution
|
- Specific conductivity of N/35 KCl at 298K is 0.002768 ohm^-1 cm^-1 and...
Text Solution
|
- The molar conductance of KCl solution at different concentration at 29...
Text Solution
|
- The conductance of 0.1 M acetic acid at 298 K is 5.20 and that of 0.00...
Text Solution
|
- The specific conductance of a saturated solution of AgCl at 298K is fo...
Text Solution
|
- The lamda^(@) values of KNO(3) and LiNO(3) are 145.0 and 110.1 S cm^(2...
Text Solution
|
- The conductivity of a 0.01M solution of acetic acid at 298K is 1.65xx1...
Text Solution
|
- The molar conductivity at infinite dilution for NH4Cl, NaOH and NaCl k...
Text Solution
|