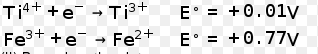Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-ELECTROCHEMISTRY-EXERCISE
- Give the Nernst equation of the cell: Ni(s)|NI^(2+)(aq)(0.1M)||Ag^(+)(...
Text Solution
|
- Calculate the standard cell potentials of galvanic cell in which the f...
Text Solution
|
- On the basis of the standard electrode potential values states for aci...
Text Solution
|
- What type of a battery is lead storage battery? Write the anode and th...
Text Solution
|
- How is molar conductivity related to conductivity of a solution? Deriv...
Text Solution
|
- Given that the standard electrode potentials of metals are: K^+|K=-2.9...
Text Solution
|
- Two half reactions in electochemical cell are given below: MnO4^-(aq)+...
Text Solution
|
- State and explain Kohlrausch's law. How would you determine the molar ...
Text Solution
|
- Calcualate the DeltarG for the reaction: Mg(s)+Cu^(2+)(aq)rarrMg^(2+)(...
Text Solution
|
- Which cell was used in Apollo space programme ? What was the product u...
Text Solution
|
- Define the following: Fuel Cell.
Text Solution
|
- Define the following: Limiting molar conductivity
Text Solution
|
- Define the following terms: Molar conductivity(Lambdam)
Text Solution
|
- Define the following terms: Secondary batteries.
Text Solution
|
- Following reactions occurs at cathode during the electrolysis of aqueo...
Text Solution
|
- Why does potential of mercury cell remain constant throughout its life...
Text Solution
|
- Two electrolytic cells containing silver nitrate solution and dilute s...
Text Solution
|
- Two electrolytic cells containing silver nitrate solution and dilute s...
Text Solution
|
- Give reasons: Rusting of iron pipe can be prevented by joining with a ...
Text Solution
|
- Give reasons: Conductivity of an electorlyte solution decreases with t...
Text Solution
|