A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-ELECTROCHEMISTRY-EXERCISE
- Find the charge in coulombs on 1g ion of N^(-3)
Text Solution
|
- A current of 3A was passed through a solution of AuCl4^- ions using go...
Text Solution
|
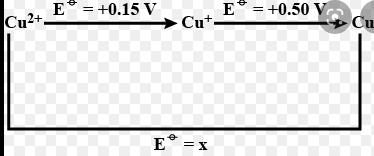
- In the diagram given below the value of x is
Text Solution
|
- By how much will the potential of a zinc electrode change if the solut...
Text Solution
|
- The e.m.f of the cell: Cu(s)|Cu^(2+)(1M)||Ag^(+)(1M)|Ag is 0.46V. The ...
Text Solution
|
- In infinite dilution of aqueous solution of BaCl2 molar conductivity o...
Text Solution
|
- Specific conductance of 0.1M sodium chloride solution is 1.06×10-2 ohm...
Text Solution
|
- Saturated solution of KNO3 with agar-agar is used to make 'salt bridge...
Text Solution
|
- Aluminium displaces hydrogen fromacids, but copper does not. A galvani...
Text Solution
|
- Given that the standard electrode potentials of metals are: K^+|K=-2.9...
Text Solution
|
- The standard reduction potentials for two reactions are given below: A...
Text Solution
|
- An electric current is passed through silver voltameter connected to a...
Text Solution
|
- The e.m.f of the following Daniell cell at 298K is E1. Zn|ZnSO4(0.01M)...
Text Solution
|
- Same amount of electric current is passed thorugh solution of AgNO3 an...
Text Solution
|
- Calculate the EMF of the cell, containing nickel and copper electrodes...
Text Solution
|
- Given E(Fe^(3+)|Fe)^@=-0.36V, E(Fe^(2+)|Fe)^@=-0.439V. The value of st...
Text Solution
|
- A^@m for NaCl, HC1 and NaAc are 126.4, 425.9 and 91.0 S cm^2 mol-1 res...
Text Solution
|
- If sin^(6)theta+cos^(6) theta-1= lambda sin^(2) theta cos^(2) theta, ...
Text Solution
|
- For spontaneity of a cell, which is correct?
Text Solution
|
- The limiting molra conductivities of HCl, CH3COONa and NaCl are respec...
Text Solution
|