A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-ELECTROCHEMISTRY-EXERCISE
- The molar conductivity of a solution of a weak acid HX(0.01M) is 10 ti...
Text Solution
|
- Which cell will measure standard electrode potential of copper electro...
Text Solution
|
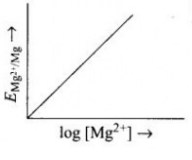
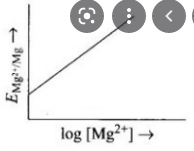
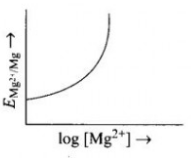
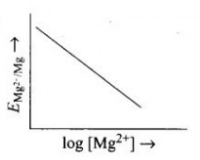
- Electrode potential for Mg electrode varies according to the equation:...
Text Solution
|
- Which of the following statement is correct?
Text Solution
|
- The differences between the electrode potentials of two electrodes whe...
Text Solution
|
- Which of the following statement is not correct about inert electrode ...
Text Solution
|
- In an electrochemical cell,
Text Solution
|
- Which is not correct
Text Solution
|
- Using the given data ,find the strongest reducing agent E^@Cr^(6+)//...
Text Solution
|
- Which of the following is the strongest oxidizing agent?
Text Solution
|
- Using this data given in Q.8 find out the which option the order of re...
Text Solution
|
- Use the data given in Q.8 and find out the most stable in its reduced ...
Text Solution
|
- Fill in the blanks- is an example of protein rich food.
Text Solution
|
- The quantity of charge requird to obtain one mole of aluminium from Al...
Text Solution
|
- Express the reation between among the conductivity of a solution in th...
Text Solution
|
- Write the chemistry of recharging the lead storage battery, highlighti...
Text Solution
|
- Lambdam^@(NH4OH) is equal to
Text Solution
|
- In the electrolysis of aq. NaCl solution which half-cell reaction will...
Text Solution
|
- Calculate the electrode potential at copper electrode dipped in a 0.1 ...
Text Solution
|
- Fill in the blanks- is an important vitamin which helps to maintain g...
Text Solution
|