Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-CHEMICAL KINETICS-EXERCISE
- The following rate data were obtained for the reaction : 2NO(g) + O2(g...
Text Solution
|
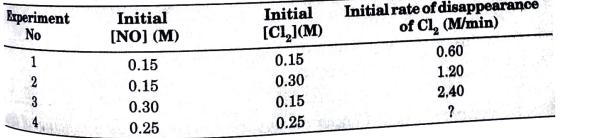
- For the reaction : 2NO(g) + Cl2(g) rarr 2NOCl(g) the following data we...
Text Solution
|
- For the reaction : 2NO(g) + Cl2(g) rarr 2NOCl(g) the following data we...
Text Solution
|
- For the reaction : 2NO(g) + Cl2(g) rarr 2NOCl(g) the following data we...
Text Solution
|
- Fill in the blanks in the following table which treats a reaction of a...
Text Solution
|
- In a reaction between A and B, the initial rate of reaction was measur...
Text Solution
|
- In a hydrolysis reaction, 5g ethyl acetate is hydr olyzed in presence ...
Text Solution
|
- A first order reaction is 20% complete in 10 minutes. Calculate the ti...
Text Solution
|
- A first order reaction takes 40 min for 30% completion. Calculate t(1/...
Text Solution
|
- 50 % of first order reaction gets completed in 16 minutes. What fracti...
Text Solution
|
- The three fourth of a first order reaction is completed in 32 minutes....
Text Solution
|
- The half life period for a reaction of first order is 2.31xx10^3 min. ...
Text Solution
|
- A reaction is first order with vosbect to reactant P having rate const...
Text Solution
|
- For a first order reaction, half life period (t(1//2)) is 100 seconds....
Text Solution
|
- The rate constant for a first order reaction is 80 s^-1. How much time...
Text Solution
|
- The pressure of a gas decomoposing at the surface of a solid catalyst ...
Text Solution
|
- The half life period of a substance is 60 min at a certain initial con...
Text Solution
|
- The thermal decomposition of a compound is first order. If 50 % of the...
Text Solution
|
- A first order reaction takes 69.3 minutes for 50% completion. Calculat...
Text Solution
|
- The rate of a reaction increases four times when the temperature chang...
Text Solution
|