Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-CHEMICAL KINETICS-EXERCISE
- For a reaction, A + B rarr Product, the rate law is given by, r = k [ ...
Text Solution
|
- First order reaction is found to have rate constant, k= 5.5xx 10^(-14)...
Text Solution
|
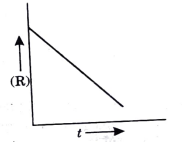
- For a chemical reaction R rarr P the variation in the concentration (R...
Text Solution
|
- For a chemical reaction R rarr P the variation in the concentration (R...
Text Solution
|
- What is difference between order of reaction and molecularity of react...
Text Solution
|
- A first order reaction has a rate constant 1.15 xx 10^-3 s^-1. How lo...
Text Solution
|
- Zero order reaction means that the rate of a reaction is independent o...
Text Solution
|
- Zero order reaction means that the rate of a reaction is independent o...
Text Solution
|
- The temperature dependence of the rate of a chemical equation can be a...
Text Solution
|
- Write down the unit of rate constant for zero order reaction.
Text Solution
|
- Give relationship between half life period and concentration for diffe...
Text Solution
|
- Write the difference between molecularity and order of reaction?
Text Solution
|
- For a first order reaction, half life period (t(1//2)) is 100 seconds....
Text Solution
|
- A reaction is first order in A and second order in B. Write the diff...
Text Solution
|
- A reaction is first order in A and second order in B How is the rate...
Text Solution
|
- A reaction is second order in A and first order in B. How is the rate ...
Text Solution
|
- A first order reaction takes 40 minutes for 30% decomposition. Calcula...
Text Solution
|
- For a first order reaction, show that time required for 99% completion...
Text Solution
|
- Deduce the rate equation for a zero order reaction.
Text Solution
|
- The half life period for the decomposition of a compound is 20 min. If...
Text Solution
|