A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-CHEMICAL KINETICS-EXERCISE
- Half life period of a first order reaction is :
Text Solution
|
- For the reaction, A rarr C , it is found that the rate of the reaction...
Text Solution
|
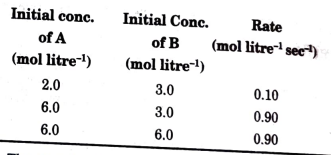
- For a chemical reaction A + B rarr C, the following data were found : ...
Text Solution
|
- On increasing temparature of the reacting system by 10 degrees, the ra...
Text Solution
|
- The rate of a first order reaction is 1.8 xx10^-3 mol L^-1 mi n^-1 whe...
Text Solution
|
- The rate for the first order reaction is 0.69 xx10^-2 mol L^-1 mi n^-1...
Text Solution
|
- For the first order reaction, the half life period is (if k is rate co...
Text Solution
|
- If k1 and k2 are rate constants at temperatures T1 and T2 respectively...
Text Solution
|
- The rate constant of a reaction is 1.2 xx10^(-5) mol^(-2)"litre"^(2)s^...
Text Solution
|
- The following rate data were obtained at 303 K for the following react...
Text Solution
|
- For a reaction X rarr Y, the rate of reaction increases by a factor of...
Text Solution
|
- For a reaction 2NO (g) + O2 (g) to 2 NO2 (g) Rate= k [NO]^2 [O2], if...
Text Solution
|
- For the first order reaction, time required for 99% completion is :
Text Solution
|
- In the reversible reaction 2 NO2 harr N2O4, the rate of disappearance ...
Text Solution
|
- For the reaction 2N2O5 to 4 NO2 + O2 rate and rate constant are 1.22 x...
Text Solution
|
- For zero order reaction, the integrated rate equation is :
Text Solution
|
- For a first order reaction involving decomposition of N2O5 the followi...
Text Solution
|
- Which of the following graphs corresponds to first order reaction:
Text Solution
|
- Which of the following is correct for a zero order reaction
Text Solution
|
- The half life period for a zero order reaction is equal to
Text Solution
|