A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-CHEMICAL KINETICS-EXERCISE
- For zero order reaction, the integrated rate equation is :
Text Solution
|
- For a first order reaction involving decomposition of N2O5 the followi...
Text Solution
|
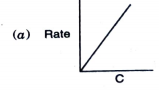
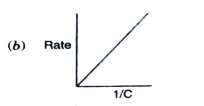
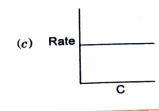
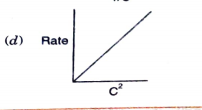
- Which of the following graphs corresponds to first order reaction:
Text Solution
|
- Which of the following is correct for a zero order reaction
Text Solution
|
- The half life period for a zero order reaction is equal to
Text Solution
|
- For a second order reaction rate at a particular time x. if the initia...
Text Solution
|
- When ln K is plotted against 1/T, the slope was found to be -10.7 xx 1...
Text Solution
|
- For a zero order reaction, linear plot was obtained for [A] vs t. The ...
Text Solution
|
- For a reaction having rate law expression Rate=k[A]^(3//2)[B]^(-1//2)....
Text Solution
|
- For a chemical reaction A rarr B ,it is observed that the rate of reac...
Text Solution
|
- The reaction -N2O5 (i n C Cl4) rarr 2NO2+1/2 O2 (g) is first order in ...
Text Solution
|
- Reaction AtoB follows second order kinetics. Doubling the concentratio...
Text Solution
|
- A reaction is 50% completes in 2 hours and 75 % completes in 4 hours. ...
Text Solution
|
- For the reaction 2N2O5 to 4 NO2 + O2 rate and rate constant are 1.22 x...
Text Solution
|
- The reaction : A rarr B follows first order kinetics. The time taken f...
Text Solution
|
- The time taken for 90% of a first order reaction to complete is approx...
Text Solution
|
- The rate law for a reaction between the Substances A and B is given by...
Text Solution
|
- In a first order reaction, the concentration of the reactant decreases...
Text Solution
|
- The rate of a first order reaction is : 1.5 xx 10^-2 mol L^-1 mi n^-1 ...
Text Solution
|
- The rate equation for the reaction 2A +B rarr C is found to be rate =k...
Text Solution
|